
The Transfusion Medicine RFID Consortium today announced that S3Edge would exclusively be leading the commercialization* efforts of the Blood Center and Hospital applications that have been designed and built by the consortium members (BloodCenter of Wisconsin, SysLogic, Inc., S3Edge Inc., Carter Bloodcare, Mississippi Blood Services, the University of Iowa Hospitals and Clinics, Mississippi Baptist Health System, University of Wisconsin Madison – RFID Laboratory, and Mediware Corp.) over the last 5 years.
- Read the entire press-release here
- Goto http://www.s3edge.com/healthcare.php to learn more
The applications that have been piloted at the BloodCenter of Wisconsin & the University of Iowa Hospitals and Clinics with promising results will help Blood Centers & Hospital Blood Banks worldwide enforce compliance with standard operating procedures while improving operational efficiency across operations in Blood Centers and Hospital blood banks.
Some highlights from the Press-release:
 “Thanks to the outstanding work of the entire consortium team, we have successfully piloted the new system to track blood products as they move from fixed and mobile donation sites, through the blood center and to distribution. After 24 weeks of running the system in a pilot mode here at the BloodCenter of Wisconsin, we have seen process efficiency and traceability gains, as well as marked improvements in reconciliation”, said Lynne Briggs, Vice President and Chief Information Officer for BloodCenter of Wisconsin in Milwaukee.
“Thanks to the outstanding work of the entire consortium team, we have successfully piloted the new system to track blood products as they move from fixed and mobile donation sites, through the blood center and to distribution. After 24 weeks of running the system in a pilot mode here at the BloodCenter of Wisconsin, we have seen process efficiency and traceability gains, as well as marked improvements in reconciliation”, said Lynne Briggs, Vice President and Chief Information Officer for BloodCenter of Wisconsin in Milwaukee.
 “The RFID system provided equivalent capabilities to those of IPR in terms of detecting and resolving process errors. It additionally provided the blood bank staff real-time visibility for blood products in transit from the hospital blood bank to the point-of-care and in remote storage in our emergency department.” said Dr. Thomas J. Raife, MD, Clinical Professor & Medical Director, UIHC DeGowin Blood Center.
“The RFID system provided equivalent capabilities to those of IPR in terms of detecting and resolving process errors. It additionally provided the blood bank staff real-time visibility for blood products in transit from the hospital blood bank to the point-of-care and in remote storage in our emergency department.” said Dr. Thomas J. Raife, MD, Clinical Professor & Medical Director, UIHC DeGowin Blood Center.
 “This effort represents the culmination of years of hard work by the consortium members in bringing a much needed innovation to the market in a collaborative manner. We are pleased to see S3Edge taking the next steps to ensure that the cumulative efforts of the consortium benefit the transfusion medicine industry as a whole”, said Rodeina Davis who was the principal investigator for the STTR grant, and a luminary in the transfusion medicine field recognized for her role in advancing this new technology from idea to adoption.
“This effort represents the culmination of years of hard work by the consortium members in bringing a much needed innovation to the market in a collaborative manner. We are pleased to see S3Edge taking the next steps to ensure that the cumulative efforts of the consortium benefit the transfusion medicine industry as a whole”, said Rodeina Davis who was the principal investigator for the STTR grant, and a luminary in the transfusion medicine field recognized for her role in advancing this new technology from idea to adoption.
 “Today’s announcement solidifies our commitment not just to create compelling technology, but to realize the vision of creating a successful product for the transfusion medicine industry. We look forward to completing the requirements for the 510(k) clearance and facilitate the technology’s potential to transform current operations in the transfusion medicine industry for all end-users” said Tina Chang, CEO of SysLogic, Inc., a Brookfield, Wisconsin-based information systems consulting and services firm.
“Today’s announcement solidifies our commitment not just to create compelling technology, but to realize the vision of creating a successful product for the transfusion medicine industry. We look forward to completing the requirements for the 510(k) clearance and facilitate the technology’s potential to transform current operations in the transfusion medicine industry for all end-users” said Tina Chang, CEO of SysLogic, Inc., a Brookfield, Wisconsin-based information systems consulting and services firm.
To learn more about the solution(s) please visit our website at www.s3edge.com , swing by the S3Edge booth (#579) at the AABB Annual Meeting & CTTXPO 2012 if you are in the Boston area from October 6-9th, 2012.or drop us a line at info@s3edge.com
Meanwhile, for the folks with a curious bent here’s a fun time-line of how this project evolved over the years and has become a beacon of successful public-private-academic partnerships can succeed with the right team…enjoy!
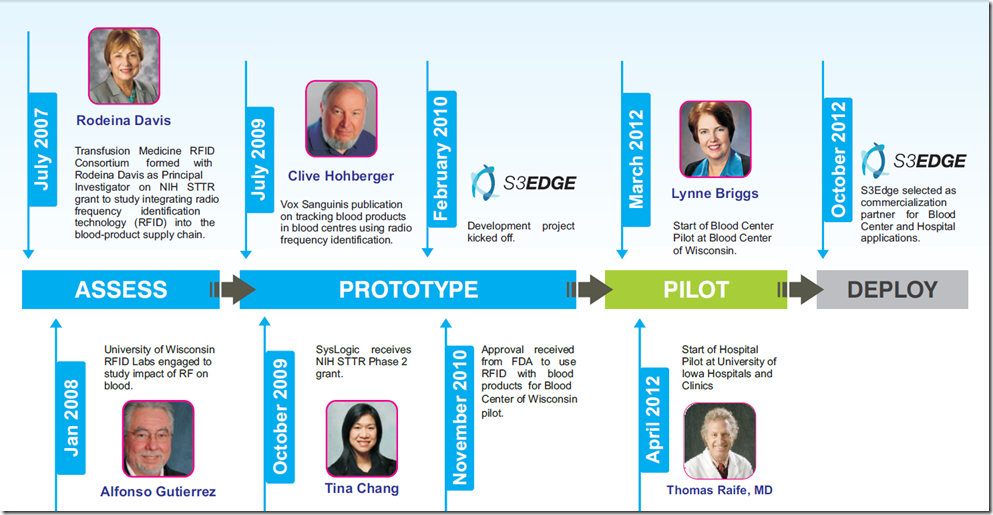
Until the post, take care and hope to see you in Boston!
Cheers
/a
*The RFID blood product tracking system is pending 510(k) submission and is not yet commercially available in the US















 “This effort represents the culmination of years of hard work by the consortium members in bringing a much needed innovation to the market in a collaborative manner. We are pleased to see S3Edge taking the next steps to ensure that the cumulative efforts of the consortium benefit the transfusion medicine industry as a whole”, said Rodeina Davis who was the principal investigator for the STTR grant, and a luminary in the transfusion medicine field recognized for her role in advancing this new technology from idea to adoption.
“This effort represents the culmination of years of hard work by the consortium members in bringing a much needed innovation to the market in a collaborative manner. We are pleased to see S3Edge taking the next steps to ensure that the cumulative efforts of the consortium benefit the transfusion medicine industry as a whole”, said Rodeina Davis who was the principal investigator for the STTR grant, and a luminary in the transfusion medicine field recognized for her role in advancing this new technology from idea to adoption.


